Introduction
Quantum physics explores how matter and energy behave at the tiniest levels, such as atoms and particles smaller than atoms. It introduces ideas like particles acting both as waves and particles, energy coming in small, fixed amounts, limits on how accurately we can measure things like position and speed, and the strange instant connections that can form between particles, no matter how far apart they are.
In the early 20th century, quantum physics completely changed our understanding of physics. Key scientists like Max Planck, Albert Einstein, and Niels Bohr played important roles in its development. This new understanding of the world at the smallest scale became the basis for many modern technologies, including semiconductors, lasers, and quantum computers.
Quantum phenomena often seem surprising and strange because they don’t match what we experience in everyday life. For instance, particles can be in more than one state at the same time (called superposition), can instantly share information across great distances (known as quantum entanglement), and can act like both particles and waves at once. The uncertainty principle also tells us that we can’t precisely measure both the position and speed of a particle at the same time. These unusual behaviors are very different from how things work in the everyday, classical world, making quantum physics both intriguing and hard to grasp.

Page URL: https://commons.wikimedia.org/wiki/
Attribute: Rosser1954, CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons
Why is Quantum Physics Important?
Quantum Physics in Everyday Technology
Semiconductors and Transistors:
In solids, quantum mechanics determines that electrons can only occupy specific energy levels. The gap between these two energy bands in semiconductors determines if a material is a conductor, semiconductor, or insulator. Semiconductors, like silicon, have a small band gap, which means electrons can jump between the bands when they receive enough energy, such as from heat or light.
Page URL: https://commons.wikimedia.org/wiki/
Attribute: The original uploader was DaRy at Swedish Wikipedia., CC BY-SA 3.0 http://creativecommons.org/licenses/by-sa/3.0/, via Wikimedia Commons
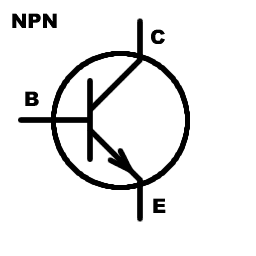
Transistors, which are the basic components of modern electronics, rely on semiconductors to manage the flow of electrons. Thanks to a process called quantum tunnelling, electrons can move through barriers that classical physics says they shouldn’t be able to cross. This allows precise control over whether electrons can move between a transistor’s source and drain terminals, effectively turning the current on or off.
By “doping” a semiconductor—adding atoms with more or fewer electrons—engineers can control the number of free electrons or “holes” (gaps where electrons are missing). This adjustment of charge carriers, which follows quantum principles, lets semiconductors switch between conducting and non-conducting states, which is how transistors work.

Page URL: https://commons.wikimedia.org/wiki/
Attribute: Tem5psu, CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons
Quantum mechanics controls how electrons move and behave in semiconductors, enabling the creation of switches (transistors) that can turn current on and off. These transistors are the foundation of all digital electronics, powering devices like smartphones and computers.
Lasers:
When an electron in an excited state moves to a lower energy level, it releases a photon (a particle of light). If this photon interacts with another excited electron, it can cause the second electron to emit a photon with the same phase, direction, and wavelength, which amplifies the light.
Normally, most electrons in atoms stay in their lowest energy state. To create a laser, energy is pumped into a material using quantum mechanics, causing more electrons to move into excited states. This “population inversion” sets the stage for these electrons to release photons in sync, producing the focused light of a laser.

Page URL: https://commons.wikimedia.org/wiki/
Attribute: 彭嘉傑, CC BY 2.5 https://creativecommons.org/licenses/by/2.5, via Wikimedia Commons
Lasers play a key role in transmitting data through fiber-optic cables. In fiber-optic communications, lasers produce beams of focused, coherent light that can carry large amounts of data, such as internet traffic and phone calls, over long distances with very little loss. This fast and high-capacity technology is the foundation of the internet’s communication network.
Quantum computing:
Quantum computing leverages the principles of quantum mechanics to perform computations in ways that classical computers cannot.
Unlike classical bits, which are strictly either 0 or 1, quantum bits (qubits) can be in a state of 0, 1, or both at the same time. This ability lets quantum computers explore many possibilities simultaneously, greatly increasing their processing power.
Qubits can be entangled, which means the state of one qubit is connected to the state of another, regardless of how far apart they are. This connection allows quantum computers to perform complex calculations more efficiently.

Page URL: https://commons.wikimedia.org/wiki/
Attribute: Ragsxl, CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons
Quantum algorithms use quantum gates to control qubits. These gates perform operations by taking advantage of superposition and entanglement, which allows quantum computers to run complex algorithms.
Quantum algorithms can solve some problems much faster than classical algorithms. For instance, Shor’s algorithm can factor large numbers exponentially faster than the best classical methods. This has important implications for cryptography, as it could potentially break many of the encryption techniques used today.
Quantum computers could potentially break widely used encryption methods, like RSA encryption, by efficiently factoring large numbers and solving discrete logarithm problems—tasks that are very difficult for classical computers.
On the other hand, quantum computing is also pushing the development of new cryptographic methods that can withstand quantum attacks. Examples include lattice-based cryptography and quantum key distribution, which are designed to be secure even against powerful quantum computers.
Quantum Sensors:
Magnetoencephalography (MEG) uses superconducting quantum interference devices (SQUIDs) to measure the magnetic fields generated by brain activity. This technique provides high-resolution maps of brain functions and helps diagnose neurological disorders.
Quantum dots are tiny semiconductor particles that have special optical properties because of quantum confinement. They can be used as sensors for highly sensitive imaging of cellular and molecular processes, which helps in the early detection and diagnosis of diseases.
Quantum-enhanced sensors can detect individual molecules with great accuracy. This ability is valuable for identifying specific biomarkers at the early stages of diseases, such as cancer.
Quantum gravimeters measure gravitational fields with remarkable precision. They use atom interferometry, a quantum technique, to detect tiny changes in gravity. This can help explore underground structures and find mineral deposits.
Quantum sensors can detect magnetic fields with high sensitivity. This is useful for identifying geological formations and mineral resources. They are also applied to monitor changes in the Earth’s magnetic field, which can be related to volcanic activity and other geological events.
Quantum accelerometers measure acceleration with great precision. They are used to study seismic activity and tectonic movements, helping to understand the Earth’s interior and monitor geological events.
In Chemistry and Biology:
Quantum mechanics explains how electrons are organized in atoms and molecules by describing orbitals—areas where electrons are most likely to be found. The way these electrons are arranged in orbitals determines an atom’s chemical properties and how it bonds with other atoms.
When atoms bond, their atomic orbitals merge to create molecular orbitals. Quantum mechanics explains how these orbitals overlap and interact to form bonds. This process is governed by principles like the Pauli exclusion principle, which says no two electrons can have the same set of quantum numbers, and Hund’s rule, which describes how electrons fill orbitals in a way that maximizes unpaired electrons.
Quantum theory explains the formation of bonding and antibonding orbitals when atoms combine. Bonding orbitals lower the system’s energy and help stabilize the molecule, while antibonding orbitals raise the energy and can destabilize the molecule if they are occupied by electrons.
Quantum mechanics sheds light on reaction mechanisms by explaining how electrons are transferred or shared during chemical reactions. It helps us understand how bonds break and form at the molecular level, providing a deeper understanding of the processes involved in chemical reactions.
Quantum mechanics helps in modeling molecular dynamics by simulating the movements of atoms and molecules over time. This allows for predicting reaction rates and outcomes by analyzing how molecules interact and change during a reaction.
Conclusion
In conclusion, quantum mechanics is fueling groundbreaking advancements in technology, with emerging fields like the quantum internet and quantum AI set to transform various industries. The quantum internet offers ultra-secure, instantaneous communication using entanglement and quantum cryptography, while quantum AI harnesses the power of quantum computing to improve data processing, optimization, and machine learning. These innovations could revolutionize sectors like telecommunications, cybersecurity, healthcare, and finance, pushing the limits of computing and communication. As these technologies evolve, they hold incredible potential to reshape the future of science, industry, and daily life.
Share the knowledge with